✔ 100% Authentic Product
👁️ Currently Viewing 1468
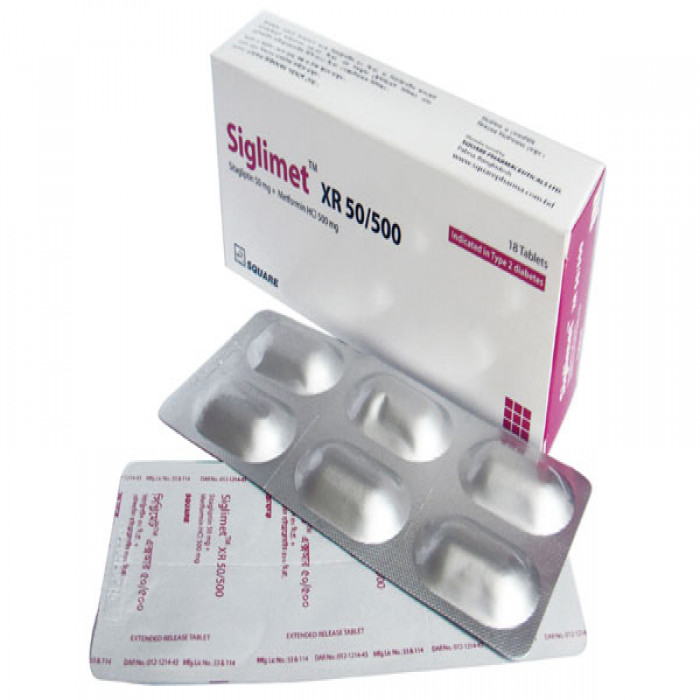
Siglimet XR 50/500mg 6pcs
Generic Name: Sitagliptin 50mg + Metformin Hydrochloride 500mg
Manufacturer/Distributor: Square Pharmaceuticals Ltd.
Discount
Price: ৳ 90
MRP:
৳
96
6%
Off

100% Genuine Products, Guaranteed

Safe & Secure Payments, Always

Fast, Secure & Efficient Delivery

Proper Packaging
 Cash on Delivery - All over Bangladesh
Cash on Delivery - All over Bangladesh Regular Delivery - 12-24 Hours, Dhaka City* Charge Tk.39-59
Regular Delivery - 12-24 Hours, Dhaka City* Charge Tk.39-59 Regular Delivery - 24-48 Hours, Other Cities* Charge Tk.99-110
Regular Delivery - 24-48 Hours, Other Cities* Charge Tk.99-110
 ফ্রি ডেলিভারিঃ - ৯৯৯ টাকা+ অর্ডারে, ঢাকা
শহরে
ফ্রি ডেলিভারিঃ - ৯৯৯ টাকা+ অর্ডারে, ঢাকা
শহরে ফ্রি ডেলিভারিঃ - ২৯৯৯ টাকা+ অর্ডারে, ঢাকার
বাহিরে
ফ্রি ডেলিভারিঃ - ২৯৯৯ টাকা+ অর্ডারে, ঢাকার
বাহিরে
100% Genuine Products, Guaranteed
Safe & Secure Payments, Always
Fast, Secure & Efficient Delivery
Proper Packaging
 Cash on Delivery - All over Bangladesh
Cash on Delivery - All over Bangladesh Regular Delivery - 12-24 Hours, Dhaka City* Charge Tk.39-59
Regular Delivery - 12-24 Hours, Dhaka City* Charge Tk.39-59 Regular Delivery - 24-48 Hours, Other Cities* Charge Tk.99-110
Regular Delivery - 24-48 Hours, Other Cities* Charge Tk.99-110 ফ্রি ডেলিভারিঃ - ৯৯৯ টাকা+ অর্ডারে, ঢাকা
শহরে
ফ্রি ডেলিভারিঃ - ৯৯৯ টাকা+ অর্ডারে, ঢাকা
শহরে ফ্রি ডেলিভারিঃ - ২৯৯৯ টাকা+ অর্ডারে, ঢাকার
বাহিরে
ফ্রি ডেলিভারিঃ - ২৯৯৯ টাকা+ অর্ডারে, ঢাকার
বাহিরে
✅ Description:
Indications
Usually shown as an aide to eat less and work out to progress glycemic control in grown-ups with sort 2 diabetes mellitus when treatment with both sitagliptin and metformin is fitting. Vital confinements of use: This ought to not be utilized in patients with sort 1 diabetes or for the treatment of diabetic ketoacidosis, because it would not be effective in these settings. This has not been considered in patients with a history of pancreatitis. It is obscure whether patients with a history of pancreatitis are at expanded hazard for the improvement of pancreatitis when utilizing This.
Pharmacology
This tablet combines two antihyperglycemic specialists with complementary instruments of activity to progress glycemic control in patients with sort 2 diabetes. Sitagliptin, a dipeptidyl peptidase-4 (DPP-4) inhibitor, and Metformin HCl, a part of the biguanide lesson. Sitagliptin may be a dipeptidyl peptidase-4 (DPP-4) inhibitor, which is accepted to apply its activities in patients with sort 2 diabetes by abating the inactivation of incretin hormones. Incretin hormones, counting glucagon-like peptide-1 (GLP-1) and glucose-dependent insulinotropic polypeptide (GIP), are discharged by the digestive system all through the day, and levels are expanded in reaction to a feast. These hormones are quickly inactivated by the protein, DPP-4. The incretins are part of an endogenous framework included within the physiologic direction of glucose homeostasis.
Dosage & Administration
Dosage of film-coated tablet: This tablet's dosage should be individualized based on the patient's current regimen, effectiveness, and tolerability, while not exceeding the maximum daily dose of 100 mg sitagliptin and 2000 mg metformin. Individualized initial combination therapy and maintenance of combination therapy should be left to the discretion of the health care provider.
The suggested beginning measurements in patients not as of now treated with metformin is 50 mg sitagliptin/500 mg metformin hydrochloride twice day by day, with progressive measurements acceleration prescribed to decrease gastrointestinal side effects related to metformin. The beginning measurements in patients as of now treated with metformin ought to give sitagliptin dosed as 50 mg twice day by day (100 mg adds up to everyday dosage) and the measurements of metformin as of now being taken. For patients taking metformin 850 mg twice every day, the suggested beginning dosage of this tablet is 50 mg sitagliptin/1000 mg metformin hydrochloride twice daily. No considers having been performed specifically looking at the security and efficacy of Sitagliptin Phosphate Monohydrate INN/Metformin Hydrochloride BP in patients already treated with other verbal antihyperglycemic operators and exchanged to Sitagliptin Phosphate Monohydrate INN/Metformin Hydrochloride BP. Any alteration in the treatment of sort 2 diabetes ought to be embraced with care and suitable observing.
Cationic Drugs: Cationic drugs disposed of by renal tubular emission: Utilize with caution. Phenprocoumon: Metformin may diminish the anticoagulant impact of phenprocoumon. In this manner, near checking of the INR is recommended. Levothyroxine: Levothyroxine can diminish the hypoglycemic impact of metformin. Checking of blood glucose levels is suggested, particularly when thyroid hormone treatment is started or ceased, and the dose of metformin must be balanced on the off chance that is essential.
Interaction
Cationic Drugs: Cationic drugs disposed of by renal tubular emission: Utilize with caution. Phenprocoumon: Metformin may diminish the anticoagulant impact of phenprocoumon. In this manner, near checking of the INR is recommended. Levothyroxine: Levothyroxine can diminish the hypoglycemic impact of metformin. Checking of blood glucose levels is suggested, particularly when thyroid hormone treatment is started or ceased, and the dose of metformin must be balanced on the off chance that is essential.
Contraindications
This tablet is contraindicated in patients with:
Renal disease or renal dysfunction, e.g., as suggested by serum creatinine levels ≥1.5 mg/dL [males], ≥1.4 mg/dL [females] or abnormal creatinine clearance which may also result from conditions such as cardiovascular collapse (shock), acute myocardial infarction, and septicemia
Acute or chronic metabolic acidosis, including diabetic ketoacidosis, with or without coma.
History of a serious hypersensitivity reaction to this tablet or sitagliptin, such as anaphylaxis or angioedema.
This tablet should be temporarily discontinued in patients undergoing radiologic studies involving intravascular administration of iodinated contrast materials, because the use of such products may result in acute alteration of renal function.
Side Effects
The foremost common unfavorable responses detailed in ≥5% of patients at the same time began on sitagliptin and metformin and more commonly than in patients treated with fake treatment were loose bowels, upper respiratory tract contamination, and headache. Adverse responses detailed in ≥5% of patients treated with sitagliptin in combination with sulfonylurea and metformin and more commonly than in patients treated with fake treatment in combination with sulfonylurea and metformin were hypoglycemia and headache. Hypoglycemia was the as it were antagonistic response detailed in ≥5% of patients treated with sitagliptin in combination with affront and metformin and more commonly than in patients treated with fake treatment in combination with affront and metformin. Nasopharyngitis was the as it were unfavorable response detailed in ≥5% of patients treated with sitagliptin monotherapy and more commonly than in patients given a placebo.
Pregnancy & Lactation
Pregnancy Classification B. Because no adequate and well-controlled studies with Sitagliptin Phosphate Monohydrate INN/Metformin Hydrochloride BP or its individual components have been conducted in pregnant women, the safety of Sitagliptin Phosphate Monohydrate INN/Metformin Hydrochloride BP in pregnant women is unknown. This tablet should only be taken during pregnancy if absolutely necessary.
Precautions & Warnings
Lactic Acidosis- Lactic acidosis can happen due to metformin aggregation. The hazard increments with conditions such as sepsis, lack of hydration, an abundance of liquor admissions, hepatic insufficiency, renal impedance, and intense congestive heart failure. Symptoms incorporate discomfort, myalgias, respiratory trouble, expanding drowsiness, and nonspecific stomach trouble. Research facility variations from the norm incorporate moo pH, expanded anion hole, and hoisted blood lactate. If acidosis is suspected, cease this tablet and hospitalize the understanding immediately. Regular checking of thyroid-stimulating hormone (TSH) levels is suggested in patients with hypothyroidism. Long-term treatment with metformin has been related to diminished vitamin B12 serum levels which may cause fringe neuropathy. Observing the vitamin B12 level is suggested.
Storage Conditions
Keep under 25 degrees away from light and heat in a dry area. Keep out of children's reach.
⚠️Disclaimer:
At ePharma, we’re committed to providing accurate and accessible health information. However, all content is intended for informational purposes only and should not replace medical advice from a qualified physician. Please consult your healthcare provider for personalized guidance. We aim to support, not substitute, the doctor-patient relationship.